Abstract
Background: Multiple myeloma (MM), characterized by the proliferation of clonal plasma B-cell in the bone marrow, is the second most common hematologic malignancy in the US. MM represents the final stage in a continuum of plasma cell dyscrasias, arising from the premalignant conditions monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM). Despite therapeutic advances, MM remains incurable with the prognostic improvement observed only in a subset of patients. The variations in clinical course are largely caused by the underlying intertumoral heterogeneity between different patients. To further improve the clinical outcome of MM patients, biomarkers that can effectively stratified patients are critically needed for directing personalized treatment. In this study, we hypothesize that the magnitude of plasma B cell malignancy is a primary factor that determines the clinical phenotypes of MM patients, which can be reflected by gene expression in CD138+ cells.
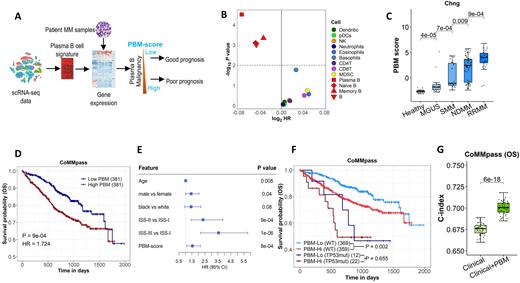
Methods: Using single-cell RNA sequencing (scRNA-seq) data, we develop a plasma B gene signature and apply it to gene expression profiles of CD138+ cells from MM patients (Figure 1 A). The resulting score, denoted as plasma B cell malignancy (PBM) score, indicates the degree of plasma B cell malignancy of MM samples with a higher value indicating higher malignancy status. Genomic and/or transcriptomic data from five MM studies (i.e., MMRF CoMMpass, UAMS, MAQC-II and APEX phase 3 trial and Chng study) were used to examine the associations between PBM score and known MM driver genes, and its clinical impact on MM progression and prognosis. Univariate and multivariate Cox regression models were constructed to evaluate the prognostic value of patient specific PBM scores when used alone or combined with established clinical factors.
Results: Survival analysis indicates that the PBM score defined based on plasma B cell markers are significantly associated with patient overall survival. In contrast, gene signatures charactering other immune cells are less (other three B cell types) or not prognostic (non-B immune cell types) (Figure 1 B). Then we investigated the effects of PBM score on MM development and prognosis. Overall, a higher PBM score was significantly associated with a more advanced stage in the spectrum of plasma cell dyscrasias (all P < 0.05, Figure 1C). Higher PBM score was also associated with shorter overall survival (OS) in patients with MM (HR = 1.72, P < 0.001) (Figure 1D) and its prognostic effect was independent of the currently available clinical prognostic model, such as the International Staging System (ISS) (Figure 1E). Mutation status of TP53 gene has been found to be associated with patient prognosis. Investigations on the relationships between PBM score and MM driver genes found that the PBM score was significantly increased in patients carrying TP53 mutations compared to those with mild-type alleles. Of note, PBM scores could predict patients’ survival even among those who don't carry TP53 mutations, with log-rank P values of 0.002 (Figure 1F). The model based on the PBM score alone had a c-index of 0.62 to predict the OS of MM. The clinical model based on age, sex, race, and (ISS) stage had a c-index of 0.67 to predict OS. Incorporating PBM score into the models based on clinical factors significantly improved the prognostic performance of the models, with the c-index increased to 0.70 for the model that combined PBM and clinical factors (P < 0.001) (Figure 1G).Conclusions: There may be a clinical utility of the PBM score to predict MM outcomes in combination with more standard clinical parameters.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal